Basic equipment and facilities in animal cell culture
Some of the specific equipment and techniques are required for the maintenance of cell cultures. A rule of thumb is that the more equipment you get, the more efficient cell culturing you perform.
Where possible, a separate room should be made available for clean cell culture work. This room should be free of through traffic and, if possible, equipped with an air flow cabinet which supplies filtered air around the work surface. A HEPA (High Efficiency Particle Air Filter) filtered air supply is desirable but not always affordable. Primary animal tissue and micro-organisms must not be cultured in or near the cell culture aboratory and the laboratory must be specifically designated for clean cell culture work. Clean laboratory coats should be kept at the entrance and should not be worn outside of this laboratory and brought back in.
If strict sterility is needed, a laminar flow hood offers the best sterile protection available. If a hazardous chemical is to be handled a Class II Biohazard Cabinet which has a vertical laminar flow should be used. However, for primary cultures and also if no laminar flow hood or sterile room is available, an area for sterile work should be set aside, where there is no thoroughfare. If aseptic techniques are adhered to and the area kept clean and tidy, sterility can be easily maintained.
All work surfaces, benches and shelves and the base of the airflow cabinets must be kept clean by frequent swabbing with 70% ethanol or an alternative disinfectant. If an airflow cabinet cannot be provided, the culture work may be done on a clean bench using a Bunsen burner to create a sterile 'umbrella' under which the work can be done.
In addition to an airflow cabinet and benching which can be easily cleaned, the cell culture laboratory will need to be furnished with an incubator or hot room to maintain the cells at 30-40 °C. The incubation temperature will depend on the type of cells being cultivated. Insect cells will grow best at around 30 °C while mammalian cells require a temperature of 37 °C. It may be necessary to use an incubator which has been designed to allow CO2 to be supplied from a main supply or gas cylinder so that an atmosphere of between 2-5% CO2 is maintained in the incubator.
In general, many cell lines can be maintained in an atmosphere of 5% CO2:95% air at 99% relative humidity. The concentration of CO2 is kept in equilibrium with sodium bicarbonate in the medium. Different media have differing buffering capacity. If a CO2 controlled incubator is not available, or cultures must be kept sealed in flasks (i.e., after treatment with some volatile substances), then cells may be maintained in flasks sealed after gassing with 5% CO2:95% air, or vessels kept in boxes gassed and then sealed with pressure sensitive tape. In the case of boxes, the humidity must be maintained with a dish of water.
Various media may be used so that a controlled CO2 atmosphere is not required and in this case a CO2 incubator is not necessary. Hepatocytes in primary culture are often maintained in Leibovitz L-15 medium which does not require a CO2 atmosphere, however, flasks must not be sealed (as the hepatocytes require a high O2 tension which is reduced with time in sealed ungassed vessels). Most cell lines are maintained at 36.5 °C, although some cultures, such as skin cultures may require lower temperatures. Cultured cells can generally survive lower temperatures, but rarely survive temperatures greater than 2 °C above normal, and therefore the incubator should be set to cut out at approximately 38.5 °C to prevent cell death. Incubators are designed to regulate an even temperature and this is more important than accuracy, i.e., temperature should be ±0.5 ° C. Most incubators have areas of differing temperature, therefore fan assisted incubators are preferable to help maintain even temperature distribution.
- Refrigerators and freezer (-20 ° C)
Both items are very important for storage of liquid media at 4 °C and for enzymes (e.g., trypsin) and some media components (e.g., glutamine and serum) at -20 °C.
A refrigerator or cold room is required to store medium and buffers. A freezer will be needed for keeping pre-aliquoted stocks of serum, nutrients and antibiotics. Reagents may be stored at a temperature of -20 °C but if cells are to be preserved it may be necessary to provide liquid nitrogen or a -70 °C freezer.

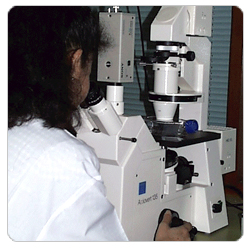
A simple inverted microscope is essential so that cultures can be examined in flasks and dishes. It is vital to be able to recognize morphological changes in cultures since these may be the first indication of deterioration of a culture. A very simple light microscope with x100 magnification will suffice for routine cell counts in a hemocytometer, although a microscope of much better quality will be required for chromosome analysis or autoradiography work.
A microscope with normal Kohler illumination will be needed for cell counting. An inverted microscope will also be needed for examining flasks and multi-well dishes from underneath. Both microscopes should be equipped with a x10 and a x20 objective and it may be useful to provide a x40 and a x100 objective for the normal microscope. Addi- tional features such as a camera, CCD video camera, adapter and attachments and UV facility may also be required for some purposes.
A variety of tissue culture plasticware is available, the most common being specially treated polystyrene. Although all tissue culture plasticware should support cell growth adequately, it is essential when using a new supplier or type of dish to ensure that cultures grow happily in it. The tests to ensure this, such as growth curves and time of reaching a confluent monolayer, are similar, to those used to ensure that serum batches are satisfactory.
Cells can be maintained in Petri dishes or flasks (25 cm2 or 75 cm2) which have the added advantage that the flasks can be gassed and then sealed so that a CO2 incubator need not be used. This is particularly useful if incubators fail. Tissue cultureware is always chosen to match the procedure.
