Tissue culture is used as a generic term to include the in vitro cultivation of organs,tissues and cells. Originally, the term is not limited to animal cells, but includes the in vitro cultivation of plant cells. Tissue culture can be subdivided into three major categories; organ culture, explants culture, and cell culture.
Organ Culture
There are disadvantages to organ cultures. Organs cannot be propagated so each piec of tissue can only be used once, which makes it difficult to assess the reproducibility of response. And, of course, the particular cells of interest may be very small in number in a given piece of tissue so the response produced may be difficult to detect and quantify It may not be possible to supply adequate oxygen and nutrients throughout the tissue because of the absence of a functioning vascular system, so necrosis of some cells occur fairly rapidly. This problem may be ameliorated to some extent by keeping the organ in stirred cultures or in roller bottles which alternately provide air and soluble nutrients.

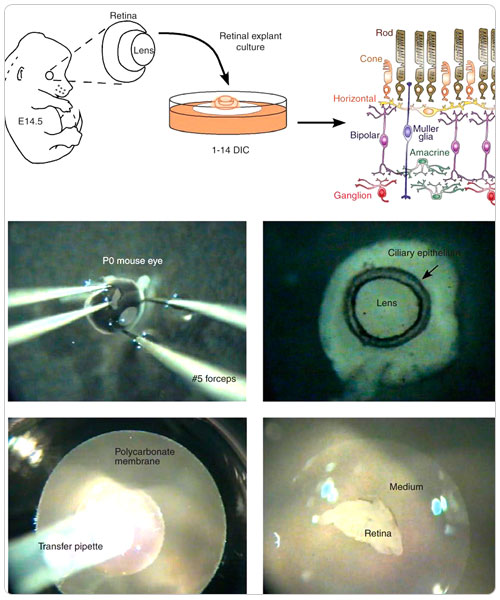
Explant (or organotypic) culture
In explant culture, small pieces of the tissue of interest are simply allowed to attach to an appropriate substrate, usually one that has been coated with collagen, and are cultured in a rich medium, usually one containing serum. Following attachment, cell migration is promoted in the plane of the solid substrate. Traditionally, explants have been maintained in Maximov chambers in which cells are grown on coverslips sealed over a depression in a thick glass slide, and this approach is still in use. More recently, it has become common to use regular culture dishes, which are much more convenient since they do not need to be disassembled and reassembled at each feeding. As with dissociated cell culture, immature tissue grows best, and explants are generally prepared from embryonic or neonatal tissue. Typically, the tissue is cut with scalpels into slices 0.5 to 1.0 mm thick, but in some cases it is simply fragmented by passing through a nylon mesh. The need for diffusion of nutrients and oxygen to the center of the explant limits thickness to about a millimeter.
In experienced hands, explant cultures can be maintained for months, and cells within the explants continue their development more or less appropriately. One of the principal advantages of this method is that some aspects of the tissue's architecture can be preserved within the explant.
Cell culture
Cell culture refers to cultures derived from dissociated cells taken from the original tissue ('primary cell culture'). Cells are dispersed (mechanically and/or enzymatically) into a cell suspension which may then be cultured as a monolayer on a solid substrate, or as a suspension in the culture medium. These cultures have lost their histotypic architecture and often some of the biochemical properties associated with it. However, they can be propagated and hence expanded and divided to give rise to replicate cultures.
Cell cultures can be characterized and a defined population can be preserved by freezing. The most obvious advantage of cell culture, and of dissociated cell culture in particular, is that it makes individual living cells accessible. All in all, primary dissociated cell cultures are particularly amenable to study using morphological and physiological techniques, which can be applied on a cell by cell basis. They are obviously less well suited to traditional biochemical approaches because the quantity of material obtainable from these cultures is usually limited and they contain a heterogeneous population of cells.
One final drawback of working with primary cell cultures is that success is not automatic. Finding the conditions that permit good cell growth and maturation, getting culture to grow reproducibly, and documenting that you have accomplished all of this entails plenty of hard work.